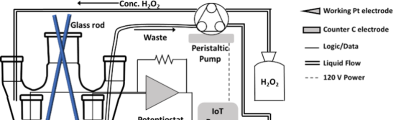
Regulatory Science Tools Catalog
The Regulatory Science Tools Catalog provides a peer-reviewed resource for use where standards and qualified Medical Device Development Tools (MDDTs) do not yet exist. These tools do not replace FDA-recognized standards or MDDTs. This catalog collates a variety of regulatory science tools that the FDA's Center for Devices and Radiological Health's (CDRH) Office of Science and Engineering Labs (OSEL) developed. If you are considering using a tool from this catalog in your marketing submissions, note that these tools have not been qualified as Medical Device Development Tools and the FDA has not evaluated the suitability of these tools within any specific context of use. You may request feedback or meetings for medical device submissions as part of the Q-Submission Program.