Catalog of Regulatory Science Tools to Help Assess New Medical Devices
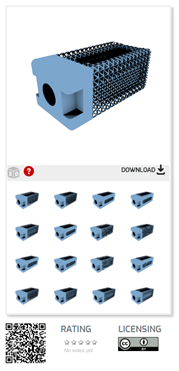
This regulatory science tool presents a computer model that consist of 16 mock intervertebral body fusion device designs with three lattice structure implementations that can enable the development of workflows, such as testing, manufacturing, and modeling, with a documented device geometry.
Technical Description
“Additively Manufactured Mock Spine Cage Designs for Mechanical Performance Test Assessments: STL Set 1” provides 16 mock intervertebral body fusion device (that is, a spinal cage) designs with three lattice structure implementations. Designs are provided in stereolithography (STL) file format so they may be fabricated using additively manufacturing (AM) technologies.
The mock cages are designed to be dimensionally representative of common posterior lumbar interbody fusion (PLIF) devices. These open-source designs are intended to assist in AM system validation and benchtop mechanical performance assessments. Examples of use include initial evaluations of new test methods, trainings on performance test methods (for example ASTM F2077), and AM system calibration pieces.
The user should verify the dimensional fidelity of the final component to that of the provided STL. This may be accomplished via optical microscopy, 3D structured light scanning, or µCT scanning.
Types of STL Files Included
Below is a description of the STL files included in this tool.
The STLs are open and non-proprietary design.
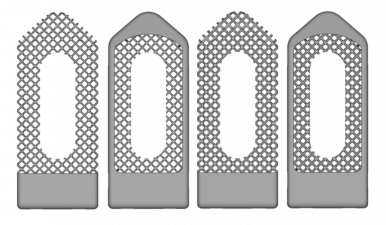
Regular Body Center Cubic (BCC)
Description:
- Designs with and without surrounding frame.
- Designs with relative densities of 0.2 and 0.3
Stochastic Voronoi Tessellation Method (VTM)
Description:
- Designs with and without surrounding frame.
- Designs with relative densities of 0.2 and 0.3
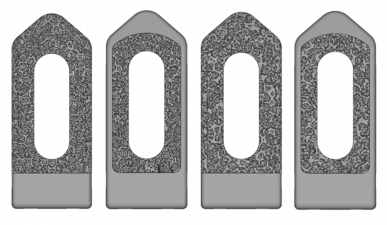
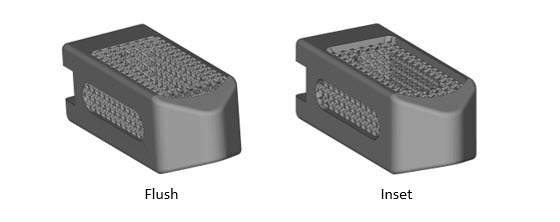
Flush Lattice Versions of the Mock Cages
Some of the cages also come in a flush (lattice is parallel to solid frame) configuration whereas the above designs have a slight lattice inset (lattice is offset from the solid frame). Below are some images of the flush lattice design.
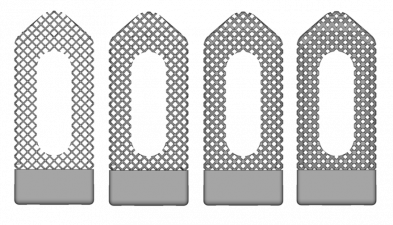
Gradually Increasing Relative Density Designs
Description:
- Each design has a monotonically increasing relative density.
- Values of 0.15, 0.25, 0.35, and 0.45
The rear of the cage has an M4 inserter design to accommodate M4 x 0.7 or M4 x 0.75 threads per mm. Inserter threads require machining (3.3 mm diameter, 3.5 mm deep feature).
Intended Purpose
The mock spinal cage designs enable stakeholders to develop workflows, such as testing, manufacturing, and modeling, with a documented device geometry. The mock devices may be used for:
- Vetting of additive manufacturing technology
- Accelerating testing methods
- Assessing benchtop methods
- Training on spine cage / AM test methods (for example, ASTM F2077-18 Test Methods for Intervertebral Body Fusion Devices)
- Refining modeling practices
- Validation of processes
This tool should be used with AM systems that can replicate the required features of the lattices. The user should confirm that manufacturing residual material is not sufficiently present in the mock devices final finished form. Residual manufacturing material may, as an example, alter the performance of the mock device by inflating the stiffness of the structure.
Testing
Additively Manufactured Mock Spine Cage Designs for Mechanical Performance Test Assessments: STL Set 1 described or used in peer reviewed publications:
Snodderly, K. L., et al. (2022). "Dimensional variability characterization of additively manufactured lattice couponsExternal Link Disclaimer." 3D Print Med 8(1): 14. DAM-5459
The mock devices in Snodderly et al. were fabricated in Ti-6Al-4V on Laser and Electron Beam Melting Powder Bed Fusion Systems.
User-specific validation will be required based on the mock device’s context of use. If the mock devices are used to evaluate the effect of mechanical performance test, then it is recommended that the AM system’s material performance variability be known to understand how to better interpret results and conclusions.
Limitations
These designs, even when fabricated, are not actual medical devices, are not intended for clinical use, are not intended for implantation, and cannot be used as predicate devices for premarket regulatory submissions. This tool is also not intended to replace testing of a U.S. legally marketed predicate device in a regulatory submission.
AM technologies and workflows used to fabricate the mock devices can alter the bench performance.
Supporting Documentation
Supporting Documentation Device designs can be accessed from NIH 3D - Additively Manufactured Mock Spine Cage Designs for Mechanical Performance Test Assessments: STL Set 1.
Contact
Tool Reference
- RST Reference Number: RST24OP02.01
- Date of Publication: 05/04/2024
- Recommended Citation: U.S. Food and Drug Administration. (2024). Additively Manufactured Mock Spine Cage Designs for Mechanical Performance Test Assessments: STL Set 1 (RST24OP02.01). https://cdrh-rst.fda.gov/additively-manufactured-mock-spine-cage-designs-mechanical-performance-test-assessments-stl-set-1
